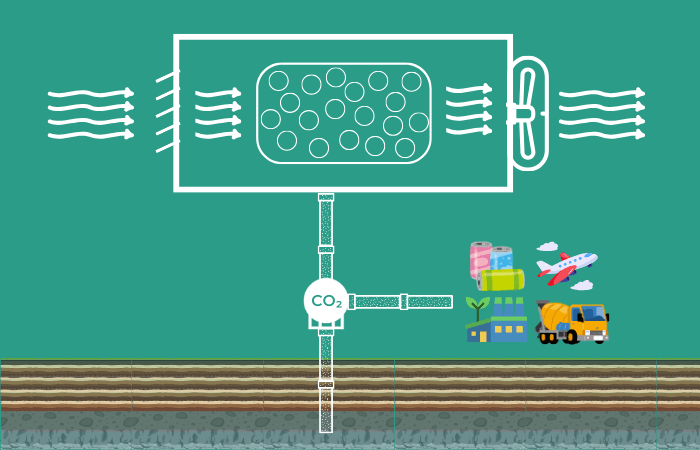
How Does Direct Air Capture Work?
In our ongoing battle against climate change, we are constantly exploring innovative technologies to reduce the concentration of carbon dioxide (CO2 ) in the atmosphere. One such technology gaining attention is Direct Air Capture (DAC) – but how does direct air capture work?
But first, what is Direct Air Capture?
Direct air capture technologies extract CO2 directly from the atmosphere by passing ambient air through a chemical process. The captured carbon dioxide can then be permanently stored in underground in geologic formation or be put to productive use in the manufacture of fuels, building materials, enhanced oil recovery and more.
How Does DAC Work?
The Two Main Approaches to Direct Air Capture
The two approaches to DAC that are the most widely used are using liquid solvents or solid adsorbents.
Solid Adsorbent DAC
This method uses solid sorbent materials that adsorb CO₂ by chemically binding with the carbon dioxide which is then released by applying heat or reducing pressure.
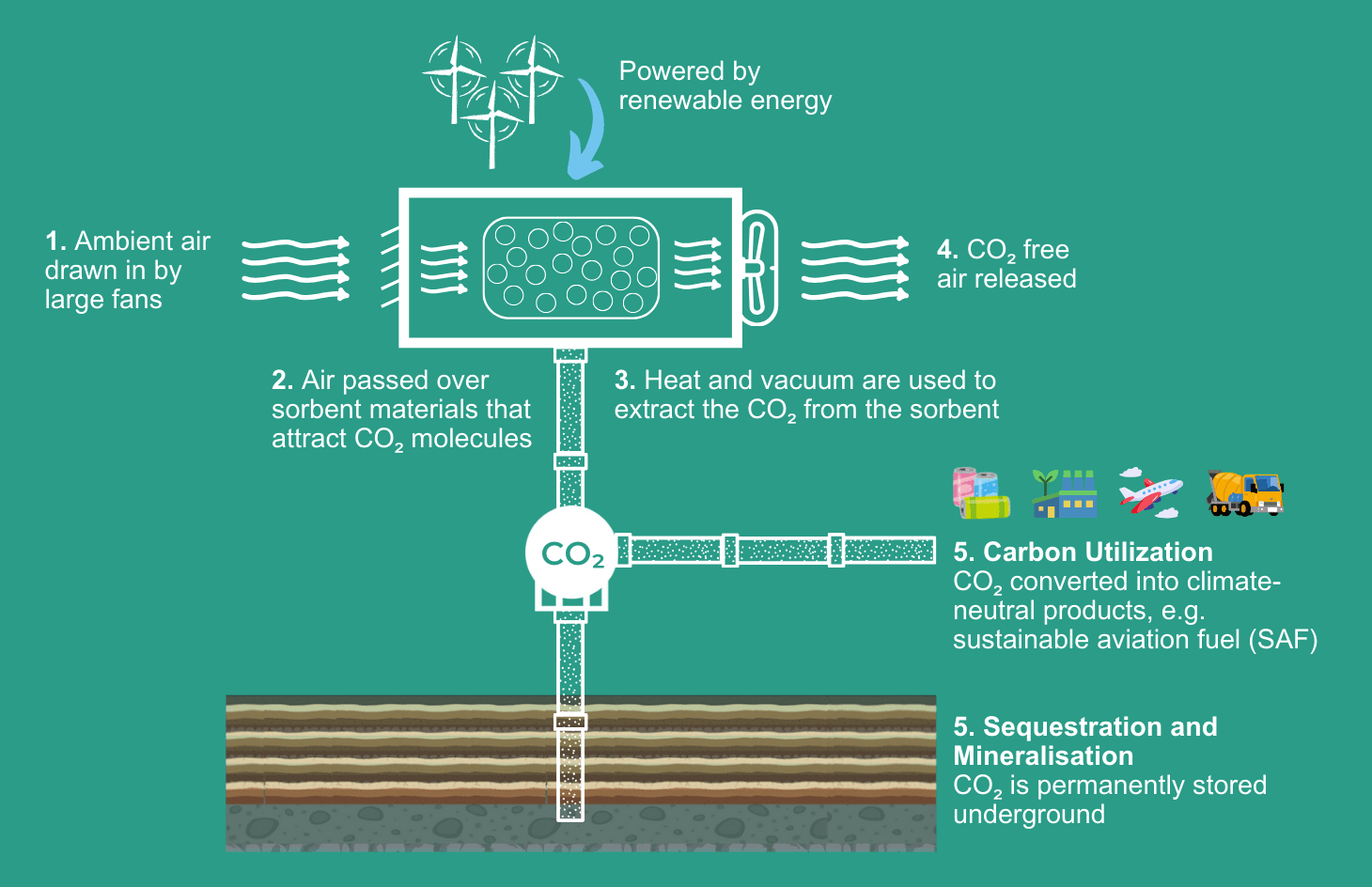
1. Air Capture:
The process begins with the extraction of air from the environment. Fans draw in large amounts of air, which contains about 0.04% CO2 by volume, along with other gases.
2. CO2 Adsorption:
The captured air is then passed through/over sorbent material which selectively binds with the CO2. This step is critical in separating CO2 from the rest of the air. Getting the choice right of adsorbent material is particularly pertinent. It has to be good at grabbing CO2, but also be able to release it again without too much energy input. Furthermore, for it to be economical, the material has to last a long time, be re-usable, and work in vastly differentiating temperatures and humidity levels.
3. Separation:
After adsorbing the CO2, the sorbent material undergoes a separation process. A vacuum is applied and then heat allows the pure CO2 to be extracted from the sorbent material. Undertaking the separation step with a low energy requirement is important to the efficiency and scale up potential of DAC. Other methods include using steam or electricity to remove the CO2.
Large amounts of pure water are also separated as part of this stage. This water is a valuable commodity that can be used in industry.
Liquid Solvent DAC
This approach uses a chemical solution, a liquid solvent like potassium hydroxide, that absorbs CO₂ by chemical reaction as the air is passed through the liquid. The CO₂ is then released by heating the solution which regenerates the solvent for reuse and allows the captured CO₂ to be collected.
What is the Difference Between “Absorption” and “Adsorption”?
“Absorption” and “adsorption” are both processes that involve how substances interact with each other, but they are quite different in how they work.
Absorption
This is when one substance is completely taken in by another. In this case, the absorbed material spreads throughout the whole volume of the absorbing substance. For example, when you pour water on a paper towel, the water gets inside the towel and spreads throughout it.
Adsorption
This is when atoms, ions or molecules stick to the surface of a material rather than getting inside. Think of it like dust sticking to a table. The molecules just stay on the outer surface and do not go deeper into the material.
In short, absorption is when something goes inside a material, while adsorption happens only on the surface of a material.
Captured Carbon Storage
Permanent CO2 Storage
There are several established routes that we could use for storing or utilizing the CO2 captured through DAC:
- Ocean storage in deep oceans to dilute into the sea water
- Geological storage: in oil and gas reservoirs, deep saline formations, and un-mineable coal beds
- Mineralization: CO2 is converted into a solid form through a process called mineral carbonation and stored virtually permanently.
Clean Air Task Force’s report highlights that there is ample potential to develop CO2 storage capacity in Europe to use carbon capture and storage:
- Europe has enough potential storage capacity to deal with projected rates of CO2 capture for at least 500 years.
- Over two thirds of European countries have enough capacity to store at least 100 years of their own captured industrial emissions.
For more, see: What is Carbon Sequestration?
NEG8 Carbon will utilize Direct Air Capture & Mineralization (DAC+M). The CO2 will be mineralized in basalt rock formations during a process where carbon dioxide is dissolved in water and interacts with reactive rock formations, such as basalts, to form stable minerals providing a permanent and safe carbon sink.
Captured Carbon Utilization
Captured carbon can be re-used in new chemical, industrial or biological applications. CO2 reuse does not replace storage, as depending on the applications it could eventually return into the atmosphere after it has been used. (For more: What is the Utilization of CO2?)
Powered by Renewable Energy
Since the DAC process is energy intensive, it has to be powered by renewable energy sources for it to be worthwhile – the likes of solar, wind and geothermal energy. Many of the Direct Air Capture systems are being located near sources of renewable energy to facilitate this important aspect of the whole process. Even more pertinent, DAC can use off-grid renewable energy that does not interfere with the energy grid of the local communities.
Conclusion
NEG8 Carbon is working hard to make the widespread adoption of DAC a reality through our modular ‘plug and play’ carbon capture units. The units have a small footprint and can be located virtually anywhere on the planet.
As we continue to seek innovative solutions to the climate crisis, Direct Air Capture is a technology that holds immense promise. By actively removing CO2 from the atmosphere, it can help us mitigate the effects of climate change and build a more sustainable future.
For more: